✅ Designed to provide long-lasting relief for chronic dry eye, featuring a low-profile dome for enhanced patient comfort and a proprietary shaft design for easy insertion and superior punctal retention.
✅ Individually packaged and preloaded on a sterile, disposable inserter for convenience. Made from medical-grade silicone and available in four sizes to ensure the perfect fit.
✅ Your struggle to find the right punctal plug ends today. We know you will like our plugs, that’s why they are backed by our 30-day free return policy for complete peace of mind.
Product Description:
VeraPlug™ is Designed to provide a simple and effective treatment for chronic dry eye.
- Individually packaged and preloaded on a sterile, disposable inserter.
- Engineered to perfectly fit the puncta for superior retention
- Low-profile dome for enhanced patient comfort
- Proprietary shaft design for easy insertion and proper anatomic fit
- Available in four sizes
- 1 pair per box
- Made by medical grade silicone
- Single use only
- You can also purchase Punctal Plug Bulk - Non Sterile (Pack of 20)
Choose the sterile preloaded configuration for the ultimate in convenience or nonsterile bulk for increased value.
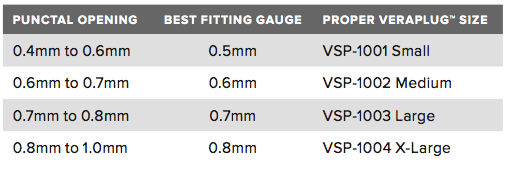
Size Chart
| PUNCTAL OPENING | VERAPLUG™ SIZE |
|---|
| 0.4mm to 0.6mm |
Small |
| 0.6mm to 0.7mm |
Medium |
| 0.7mm to 0.8mm |
Large |
| 0.8mm to 1.0mm |
X-Large |
VeraPlugTM - Instructions For Use - Please always refer to the insert in the box for latest information
Description
The VeraPlugTM punctal occluder is designed to provide reduction or elimination of tear drainage through the inferior or superior puncta, thus maintaining lubricating tears on the surface of the eye. Each VeraPlugTM punctal occluder is molded from medical grade silicone. The VeraPlugTM is available in four sizes (small, medium, large and x-large) and is packaged sterile, two per box. Each occluder is sterile, preloaded on an inserter.
Indications for Use
The VeraPlugTM is for use in patients with dry eye syndromes.
Contraindications
Contraindications include, but are not limited to, eye infections, sensitivity or allergies to the occluder material and/or materials used in the manufacture of the device, blockage/infection of the lacrimal systems, inflammation of the eyelid, and epiphora.
Precautions
The VeraPlugTM may enhance the e ect of some ocular medications in the eye. Depending on the type of medication being used the dose may need to be altered accordingly. If the patient experiences irritation, infection or epiphora after the insertion of the VeraPlug,TM the occluder should be removed.
Potential Adverse Events
The following complications may occur:
- Epiphora
- Pyogenic granuloma
- Foreign body sensation
- Infection of the lacrimal system
- Plug dislodgement or migration possibly requiring surgical intervention
- Washout
- Punctal erosion
Product Features
Each box contains two individually packaged sterile VeraPlugTM punctal occluders preloaded on inserters for single use only. The VeraPlugTM is manufactured from implant grant silicone.
Proper Sizing
The VeraPlugTM punctal occluder is available in four sizes: small, medium, large and x-large. To determine the proper size, begin with the smallest VeraPlugTM gauge (0.5mm) and insert the head of the gauge into the punctal opening. The gauge should fit snugly with a small amount of resistance. If the gauge enters the punctal opening with no resistance, the next larger size gauge should be tried in the same manner. Repeat this process until the proper size VeraPlugTM is determined based on the sizing chart shown.
Insertion
-
Anesthetize the area of the punctum with a topical anesthetic placed in the conjunctival sac.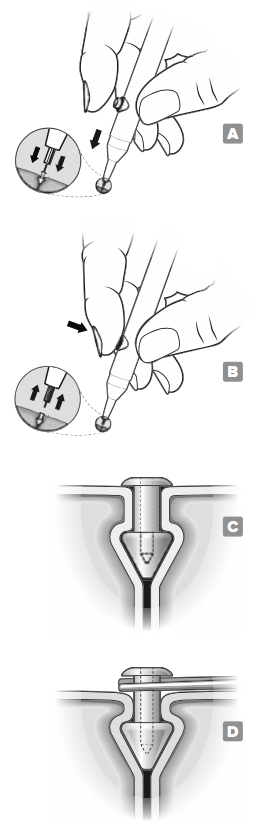
-
Apply a drop of saline solution or artificial tears onto the VeraPlugTM to help ease insertion.
-
Position the insertion instrument by placing the forefinger on the release button of the inserter and placing the occluder end of the insertion instrument over the patient's (superior or inferior) punctum.
-
Vertically insert the VeraPlugTM by positioning the occluder into the punctum until the cap is flush with the punctal opening. FIGURE A
-
When the occluder is properly seated, depress the release button and withdraw the insertion instrument. FIGURE B
-
Verify that the occluder is properly placed by confirming that the cap is flush with the punctal opening. FIGURE C After insertion, monitor the placement and integrity of the occluder to determine if/when the occluder may need to be replaced.
Removal
Should removal be indicated, grasp the vertical shaft of the occluder underneath the exposed cap with sterile forceps. Gently pull upward until the plug is removed. FIGURE D
Sterilization
VeraPlugTM sterile preloaded punctal occluders are o ered in individual trays, two per box. The date of expiration should be confirmed prior to use. If the expiration date has lapsed the occluder should be discarded.
Storage
Store at room temperature.
Warnings
The VeraPlugTM punctal occluder is intended for single use. Do not reuse. If the sterile packaging is damaged or opened sterility is not guaranteed and the VeraPlugTM should be discarded. U.S. federal law restricts the sale of this device by or on the order of a physician.
Documentation
Each box contains Instructions for Use and two labels for your ease and convenience.
Note: All products sold on Accuspire.com should be used by qualified clinician and and should be purchased by or on order of a qualified clinician.















