
Punctal Plug Pre Loaded (Sterile) 2/Box
➤ Designed to provide long-lasting relief for chronic dry eye, featuring a low-profile dome for enhanced patient comfort and a proprietary shaft design for easy insertion and superior punctal...

 US FDA Registered
US FDA Registered➤ Designed for effective dry eye relief, these FlexFit™ punctal plugs adapt to multiple sizes for a secure and comfortable fit.
➤ Preloaded & Sterile for Convenience, individually packaged for easy insertion and optimal patient safety.
➤ Trusted for its flexibility and reliability, ensuring long-lasting moisture retention. Backed by our 30-day free returns for complete peace of mind.
Product Description
Size Chart
| PUNCTAL OPENING | VERAPLUG™ SIZE |
|---|---|
| 0.2mm to 0.3mm | X-Small |
| 0.3mm to 0.5mm | Small |
| 0.6mm to 0.8mm | Medium |
| 0.9mm to 1.0mm | Large |
Description
Sterile Preloaded Punctal Occlusion System
The VeraPlugTM FlexFitTM punctal occluder is designed to provide reduction or elimination of tear drainage through the inferior or superior puncta, thus maintaining lubricating tears on the surface of the eye. Each VeraPlugTM FlexFitTM punctal occluder is molded from medical grade silicone. The VeraPlugTM FlexFitTM is available in four sizes (x-small, small, medium, and large) and is packaged sterile, two per box. Each occluder is sterile, preloaded on an inserter.
Indications for Use
The VeraPlugTM FlexFitTM is for use in patients with dry eye syndromes.
Contraindications
Contraindications include, but are not limited to, eye infections, sensitivity or allergies to the occluder material and/or materials used in the manufacture of the device, blockage/infection of the lacrimal systems, in ammation of the eyelid, and epiphora.
Precautions
The VeraPlugTM FlexFitTM may enhance the e ect of ocular medications in the eye. Depending on the type of medication being used the dose may need to be altered accordingly. If the patient experiences irritation, infection or epiphora after the insertion of the VeraPlugTM FlexFit,TM the occluder should be removed.
Potential Adverse Events
The following complications may occur:
Product Features
Each box contains two individually packaged sterile VeraPlugTM FlexFitTM punctal occluders preloaded on inserters for single use only. The VeraPlugTM FlexFitTM is manufactured from implant grade silicone.
Proper Sizing
Proper sizing can be determined by using a 0.3mm gauge. If the gauge tip is snug in the punctum, then a small size occluder is required. If there is no resistance to the gauge then the next larger size should be tried. If resistance is extremely tight, an x-small size occluder should be used.
Insertion
Anesthetize the area of the punctum with a topical anesthetic placed in the conjunctival sac.
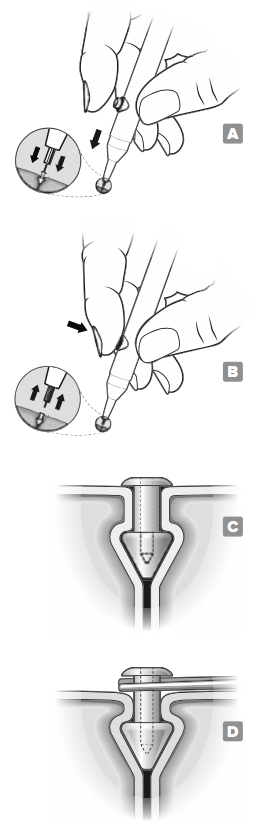
Apply a drop of saline solution or artificial tears onto the VeraPlugTM to help ease insertion.
Position the insertion instrument by placing the forefinger on the release button of the inserter and placing the occluder end of the insertion instrument over the patient's (superior or inferior) punctum.
Vertically insert the VeraPlugTM by positioning the occluder into the punctum until the cap is flush with the punctal opening. FIGURE A
When the occluder is properly seated, depress the release button and withdraw the insertion instrument. FIGURE B
Verify that the occluder is properly placed by confirming that the cap is flush with the punctal opening. FIGURE C After insertion, monitor the placement and integrity of the occluder to determine if/when the occluder may need to be replaced.
Removal
Should removal be indicated, grasp the vertical shaft of the occluder underneath the exposed cap with sterile forceps. Gently pull upward until the plug is removed. FIGURE D above
Sterilization
VeraPlugTM FlexFit sterile preloaded punctal occluders are o ered in individual trays, two per box. The date of expiration should be confirmed prior to use. If the expiration date has lapsed the occluder should be discarded.
Storage
Store at 15-30° Celsius.
Warnings
The VeraPlugTM FlexFit punctal occluder is intended for single use. Do not reuse. If the sterile packaging is damaged or opened sterility is not guaranteed and the VeraPlugTM should be discarded. U.S. federal law restricts the sale of this device by or on the order of a physician.
Documentation
Each box contains Instructions for Use and two labels for your ease and convenience.
Note: All products sold on Accuspire.com should be used by qualified clinician and and should be purchased by or on order of a qualified clinician.
Interesting Historical Information
History and Development of Punctal Plugs
1. Origins: The concept of punctal occlusion has been practiced for decades, initially using surgical techniques or improvised materials like cauterization or sutures. Punctal plugs as we know them today were developed in the 1970s and 1980s, coinciding with increased awareness of dry eye syndrome and advances in polymer technology.
2. Milestones: The first silicone punctal plugs were introduced as a minimally invasive, reversible alternative to punctal cautery. Absorbable plugs emerged as a solution for temporary tear retention, allowing patients and doctors to evaluate the effectiveness of punctal occlusion before committing to permanent plugs.
3. Modern Advancements: Innovations include bio-degradable materials, extended-duration plugs, and advanced designs for easier insertion and better patient comfort. Plugs are now available in a range of sizes and materials to suit different anatomical needs and tear drainage conditions.
Interesting Facts About Punctal Plugs
1. Reversible Treatment: Punctal plugs provide a non-surgical, reversible alternative to permanent tear drainage occlusion techniques like cautery.
2. Custom Fit: Plugs come in various diameters to fit the punctal anatomy precisely, ensuring effective tear retention without discomfort.
3. Minimal Invasiveness: The insertion procedure is quick and painless, often performed in a clinic setting with minimal preparation.
4. Improved Compliance: Patients often find punctal plugs more convenient than frequent use of artificial tears, especially for severe dry eye cases.

Discounted Prices